When regulations change, new challenges pop up. This time, it meant that our client was at a crossroads where both paths led to the market but one being significantly more elaborate and costly than the other. The only way to defend the easier path would be to know that no nanoparticles are released by their medical device products in biological fluids. Otherwise, an accurate characterization in terms of their nature, size, and concentration would be needed.
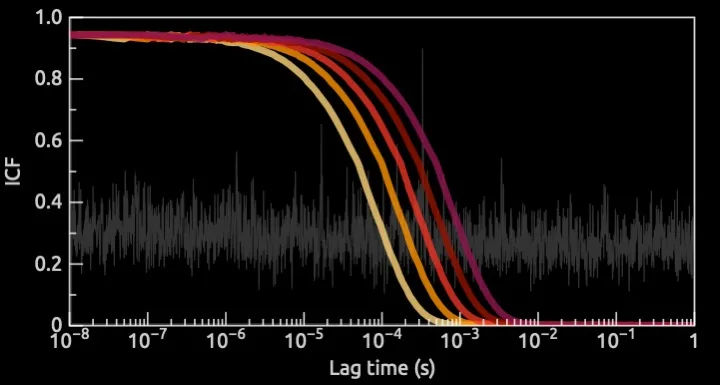
To help them, we performed dynamic and static light scattering (DLS/SLS) measurements with a state-of-the-art instrumentation. With the combination we had a high degree of freedom to choose the right experimental parameters but more importantly we could assess the raw data to correctly interpret the results. With many commercial instruments, that is not possible.
We first started with some quick feasibility measurements, to verify that we could obtain a signal and optimize the experimental parameters, and therefore to confirm that DLS/SLS was the right technique to give our client an answer.
Then, by using a standard of nanoparticles related to the product, with known concentration and size, we were able to determine the detection limit of the technique for those experimental conditions. Finally, we studied the release process from their actual products as a function of time or depending on the biological fluid they were exposed to.
As a result, we could state that the amount of nanoparticles released was lower than the detection limit in different relevant conditions. By providing this information, we guided our client to the correct road to comply with the new regulations: it happened to be the easier one, and they were now able to defend it properly.